Vaccine Cold Chain
COVID-19 vaccine products may impact the types of vaccine storage units and temperature monitoring devices used to maintain the cold chain, including the use of ultra-cold storage. Temperature ranges for COVID-19 vaccines may also differ from those for other vaccines. Carefully review the COVID-19 vaccine storage and handling addendum for information about which storage units and monitoring devices are appropriate, including specifications for monitoring devices that can monitor ultra-cold temperatures, how best to monitor temperatures, and how a specific vaccine product’s cold chain requirements may affect other vaccines in a storage unit.
Proper vaccine storage and handling play critical roles in efforts to prevent vaccine-preventable diseases. Vaccines exposed to storage temperatures outside the recommended ranges may have reduced potency, creating limited protection and resulting in the revaccination of patients and thousands of dollars in wasted vaccine.
Proper storage and handling begin with an effective vaccine cold chain.
A cold chain is a temperature-controlled supply chain that includes all vaccine-related equipment and procedures. The cold chain begins with the cold storage unit at the manufacturing plant, extends to the transport and delivery of the vaccine and correct storage at the provider facility, and ends with administration of the vaccine to the patient.
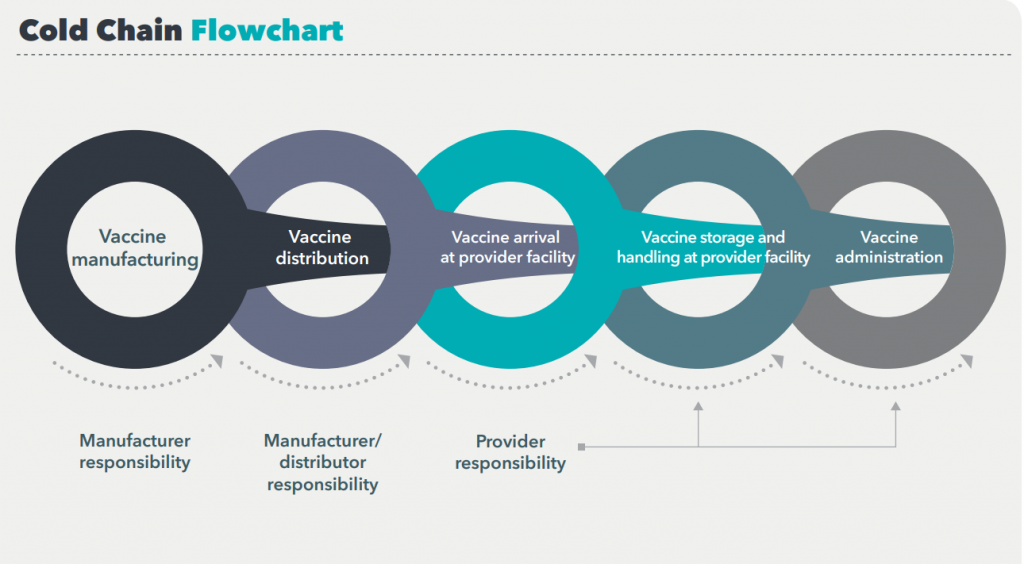
If the cold chain is not properly maintained, vaccine potency may be lost, resulting in a useless vaccine supply
Vaccines must be stored properly from the time they are manufactured until they are administered. Potency is reduced every time a vaccine is exposed to an improper condition. This includes overexposure to heat, cold, or light at any step in the cold chain. Once lost, potency cannot be restored.
Exposure to any inappropriate conditions can affect potency of any refrigerated vaccine, but a single exposure to freezing temperatures (0° C [32° F] or colder) can actually destroy potency. Liquid vaccines containing an adjuvant can permanently lose potency when exposed to freezing temperatures.
When the cold chain fails
Ensuring vaccine quality and maintaining the cold chain are shared responsibilities among manufacturers, distributors, public health staff, and health care providers.
An effective cold chain relies on three main elements:
- A well-trained staff
- Reliable storage and temperature monitoring equipment
- Accurate vaccine inventory management
Results of a cold chain failure can be costly.1,2,3 ACIP’s General Best Practice Guidelines for Immunization states, “vaccine exposed to inappropriate temperatures that is inadvertently administered should generally be repeated.”
A break in the cold chain can mean extra doses for patients, increased costs for providers, and damage to public confidence in vaccines.
More importantly, patients refusing revaccination can remain unprotected from serious, vaccine-preventable diseases.
Vaccine appearance is not a reliable indicator that vaccines have been stored in appropriate conditions
For example, inactivated vaccines—even when exposed to freezing temperatures—may not appear frozen, giving no indication of reduced or lost potency.
By following a few simple steps and implementing CDC-recommended storage and handling practices, providers can ensure patients receive high-quality vaccines that have not been compromised.
- Department of Health and Human Services, Office of Inspector General. Vaccines for Children Program: Vulnerabilities in Vaccine Management, June 2012, oig.hhs.gov/oei/reports/oei-04-10-00430.asp.
- Gazmararian JA, Oster NV, Green DC, Schuessler L, Howell K, et al. Vaccine storage practices in primary care physician offices: assessment and intervention. Am J Prev Med 2002;23(4):246–53.
- Bell KN, Hogue CJR, Manning C, Kendal AP. Risk factors for improper vaccine storage and handling in private provider offices. Pediatrics 2001;107(6):1–5.
- Centers for Disease Control and Prevention. ACIP’s General Best Practice Guidelines for Immunization, https://www.cdc.gov/vaccines/hcp/ acip-recs/general-recs/index.html






