Vaccine Storage and Temperature Monitoring Equipment
COVID-19 vaccine products may impact the types of vaccine storage units and temperature monitoring devices used to maintain the cold chain, including the use of ultra-cold storage. Temperature ranges for COVID-19 vaccines may also differ from those for other vaccines. Carefully review the COVID-19 vaccine storage and handling addendum for information about which storage units and monitoring devices are appropriate, including specifications for monitoring devices that can monitor ultra-cold temperatures, how best to monitor temperatures, and how a specific vaccine product’s cold chain requirements may affect other vaccines in a storage unit.
It is important your facility has proper storage and monitoring equipment that is set up correctly, maintained appropriately, and repaired as needed. This equipment protects patients from inadvertently receiving compromised vaccine and your facility against costs of revaccinating patients, replacing expensive vaccines, and losing patient confidence in your practice.
Vaccine Storage Units: Refrigerator and Freezer Recommendations
There are several types of vaccine storage units available. Purpose-built units are specifically designed to store vaccines. However, household-grade units are also an acceptable option for vaccine refrigeration under the right conditions.
- Use purpose-built or pharmaceutical-grade units designed to either refrigerate or freeze
These units can be compact, under-the-counter style or large.
Purpose-built units, sometimes referred to as “pharmaceutical grade,” are designed specifically for storage of biologics, including vaccines. These units often have:
- Microprocessor-based temperature control with a digital temperature sensor (thermocouple, resistance temperature detector [RTD], or thermistor)
- Fan-forced air circulation with powerful fans or multiple cool air vents promoting uniform temperature and fast temperature recovery from an out-of-range temperature. Household-grade units can be an acceptable alternative to pharmaceutical-grade vaccine storage units.
As the name implies, these units are primarily designed and marketed for home use. However, the freezer compartment of this type of unit is not recommended to store vaccines and there may be other areas of the refrigerated compartment that should be avoided as well. If your facility provides frozen vaccine, a separate freezer unit is necessary.
Do not store any vaccine in a dormitory-style or bar-style combined refrigerator/freezer unit under any circumstances.
These units have a single exterior door and an evaporator plate/cooling coil, usually located in an ice maker/freezer compartment. These units pose a significant risk of freezing vaccines, even when used for temporary storage. (Note: Not all small storage units are dormitory- or bar-style units. Compact, purpose-built units for biologics can be used to store vaccines.)
Storage unit doors
A door that is not sealed properly or left open unnecessarily not only affects the temperature in a unit, it also exposes vaccines to light, which can reduce potency of some vaccines. Consider using safeguards to ensure the doors of the unit remain closed—for example, self closing door hinges, door alarms, or door locks.

Storage Unit Best Practices
Storage Unit Placement
Good air circulation around the outside of the storage unit is important. Place a storage unit in a well-ventilated room, leaving space between the unit, ceiling, and any wall. Nothing should block the cover of the motor compartment. The unit should be firm and level, with the bottom of the unit above the floor. Make sure the unit door opens and closes smoothly and fits squarely against the body of the unit. If not secured properly, unit doors pose a particular risk to maintaining appropriate internal temperatures of vaccine storage units. Studies find most units work best when placed in an area with standard indoor room temperatures, usually between 20° C and 25° C (68° F and 77° F). Check the manufacturer-supplied owner’s manual for additional guidance on placement and spacing.
Stabilizing Temperatures in New and Repaired Units
It may take two to seven days to stabilize the temperature in a newly installed or repaired refrigerator and two to three days for a freezer.
Before using a unit for vaccine storage, check and record the minimum and maximum temperatures each workday for two to seven days. If temperatures cannot be recorded digitally, check and record temperatures a minimum of two times each workday. Once you have two consecutive days of temperatures recorded within the recommended range, your unit is stable and ready for use.
Temperature Ranges
Refrigerators should maintain temperatures between 2° C and 8° C (36° F and 46° F).* Freezers should maintain temperatures between -50° C and -15° C (-58° F and +5° F). Refrigerator or freezer thermostats should be set at the factory-set or midpoint temperature, which will decrease the likelihood of temperature excursions.
Consult the owner’s manual for instructions on how to operate the thermostat. Thermostats are marked in various ways and, in general, show levels of coldness rather than temperatures. The only way to know the temperature where vaccines are stored is to measure and monitor it with a temperature monitoring device.
Temperature Monitoring Device (TMD)
Every vaccine storage unit must have a TMD. An accurate temperature history that reflects actual vaccine temperatures is critical for protecting your vaccines. Investing in a reliable device is less expensive than replacing vaccines wasted due to the loss of potency that comes from storage at out-of-range temperatures.
- CDC recommends a specific type of TMD called a “digital data logger” (DDL).
A DDL provides the most accurate storage unit temperature information, including details on how long a unit has been operating outside the recommended temperature range (referred to as a “temperature excursion”). Unlike a simple minimum/maximum thermometer, which only shows the coldest and warmest temperatures reached in a unit, a DDL provides detailed information on all temperatures recorded at preset intervals.
Many DDLs use a buffered temperature probe, which is the most accurate way to measure actual vaccine temperatures. Temperatures measured by a buffered probe match vaccine temperatures more closely than those measured by standard thermometers, which tend to reflect only air temperature.
Temperature data from a DDL can either be downloaded to a computer using special software or retrieved from a website. The software or website may also allow you to set the frequency of temperature readings. Reviewing DDL data is critical for vaccine viability, so it is important to decide whether independent software or a website program works best for your facility.
- Keep the data for three years so they can be analyzed for long-term trends and/or recurring problems.
Those receiving public vaccine may need to keep records longer as required by state regulations.
- Use a DDL or other appropriate TMD for:
- Each vaccine storagge unit
- Each transport unit (emergency and non-emergency)
Have at least one backup TMD in case a primary device breaks or malfunctions.
- Use DDLs with the following features:
- Detachable probe that best reflects vaccine temperatures (e.g., a probe buffered with glycol, glass beads, sand, or Teflon®)
- Alarm for out-of-range temperatures
- Low-battery indicator
- Current, minimum, and maximum temperature display
- Recommended uncertainty of +/-0.5° C (+/-1° F)
- Logging interval (or reading rate) that can be programmed by the user to measure and record temperatures at least every 30 minutes
Use DDLs with a current and valid Certificate of Calibration Testing.
- A DDL’s Certificate of Calibration Testing should include:
- Model/device name or number
- Serial number
- Date of calibration (report or issue date)
- Confirmation that the instrument passed testing (or instrument is in tolerance)
- Recommended uncertainty of +/-0.5° C (+/-1° F) or less
To determine if a Certificate of Calibration Testing or Report of Calibration was issued by an appropriate entity, check to see if the certificate indicates one or more of the following items about calibration testing:
- Conforms to International Organization for Standardization (ISO)/International Electrotechnical Commission (IEC)
- 17025 international standards for calibration testing and traceability
- Performed by a laboratory accredited by International Laboratory Accreditation Cooperation (ILAC) Mutual Recognition Arrangement (MRA) signatory body
- Traceable to the standards maintained by the National Institute of Standards and Technology (NIST)
- Meets specifications and testing requirements for the American Society for Testing and Materials (ASTM) Standard E2877 Tolerance Class F or higher
- Refers to another acceptable accuracy validation method, such as comparison to other traceable reference standards or tests at thermometric fixed points
- Calibration testing should be done every two to three years or according to the manufacturer’s suggested timeline
TMDs can experience a “drift” over time, affecting their accuracy. This testing ensures the accuracy of the device continues to conform to nationally accepted standards.
Mishandling a TMD can affect its accuracy. If a TMD is dropped, hit against the side of a storage unit, or is potentially damaged in any way, its accuracy should be checked against another calibrated TMD. If there is any question about accuracy, the device should be replaced or sent for calibration testing.
Monitoring Vaccine Temperature and Vaccine Equipment
Monitoring vaccine storage equipment and temperatures are daily responsibilities to ensure the viability of your vaccine supply and the safety of your patients. Implementing routine monitoring activities can help you identify temperature excursions quickly and take immediate action to correct them, preventing loss of vaccines and the potential need for revaccination of patients.
Power Supply
Even with appropriate equipment and temperature monitoring practices in place, power disruption can result in destruction of the entire vaccine supply. Precautions should always be taken to protect the storage unit’s power supply.
- Plug in only one storage unit per electrical outlet to avoid creating a fire hazard or triggering a safety switch that turns the power off.
- Use a safety-lock plug or an outlet cover to prevent the unit from being unplugged.
- Post “DO NOT UNPLUG” warning signs at outlets and on storage units to alert staff, custodians, electricians, and other workers not to unplug units.
- Label fuses and circuit breakers to alert people not to turn off power to a storage unit.
- Use caution when using power outlets that can be tripped or switched off and avoid using:
- Built-in circuit switches (may have reset buttons)
- Outlets that can be activated by a wall switch
- Mult-ioutlet power strips
If built-in circuit switches or power strip surge protection must be used, make sure the power strip is rated to carry the maximum current as specified by the manufacturer of the refrigerator or freezer. Contact the unit manufacturer for any additional questions or guidance regarding circuit switches, power strips, or surge protection.
If the entire storage unit is affected by a temperature excursion because of a power supply issue or unit malfunction, refer to your facility’s emergency SOPs.

Temperature Monitoring
Organizing and Storing Vaccine
Correctly organizing and placing vaccines in a storage unit helps prevent conditions that could reduce vaccine potency or cause vaccine failure.
- Store vaccines in their original packaging with lids closed until ready for administration
Vials and manufacturer filled syringes should always be stored in their original packaging. Loose vials or syringes may be exposed to unnecessary light, potentially reducing potency, and may be more difficult to track for expiration dates. They may also impact inventory management and increase the risk of administration errors because they may be confused with other vaccines. For certain purpose-built units, it is recommended that vaccines be stored outside of the packaging. If this is the case, follow the manufacturer’s guidance for vaccine storage.
- Check and record storage unit minimum and maximum temperatures at the start of each workday
If your TMD does not read min/max temperatures, then check and record the current temperature a minimum of 2 times per workday (at the start and end of the workday).
Record:
- Minimum/maximum temperature
- Date
- Time
- Name of person who checked and recorded the temperature
- Any actions taken if a temperature excursion occurred
If a reading is missed, leave a blank entry in the log.
Temperature Excursions
Temperature excursions or inappropriate storage conditions for any vaccine require immediate action. Any temperature reading outside the recommended ranges in the manufacturers’ package inserts* is considered a temperature excursion. In general, manufacturers analyze information about the magnitude of the temperature excursion and the total amount of time that temperatures were out of range, as well as information about the vaccine in question, to determine whether a vaccine is likely to still be viable.

Organizing and Storing Vaccine
- CDC recommends the following steps in the event of a temperature excursion:
- Any staff who hears an alarm or notices a temperature excursion on the DDL should notify the primary or alternate vaccine coordinator immediately or report the problem to their supervisor.
- Notify staff by labeling exposed vaccines “DO NOT USE” and placing them in a separate container apart from other vaccines (do not discard these vaccines).
- The vaccine coordinator, supervisor, or if necessary, the person reporting the problem should begin to document the event with the following information – a) Date and time of the temperature excursion b) Storage unit temperature as well as room temperature, if available (including minimum/maximum temperatures during the time of the event, if available) c) Name of the person completing the report and description of the event.
- Implement your facility SOPs to adjust unit temperature to the appropriate range. At a minimum, check the TMD to make sure it is appropriately placed in the center of the vaccines.
- Contact your immunization program and/or vaccine manufacturer(s) per your SOPs for further guidance on whether to use affected vaccines and for information about whether patients will need to be recalled for revaccination. Be prepared to provide documentation of the event (e.g., temperature log data) to ensure you receive the best guidance.
- . Complete your documentation of the event, including a) action taken b) result

Organizing and Storing Vaccine
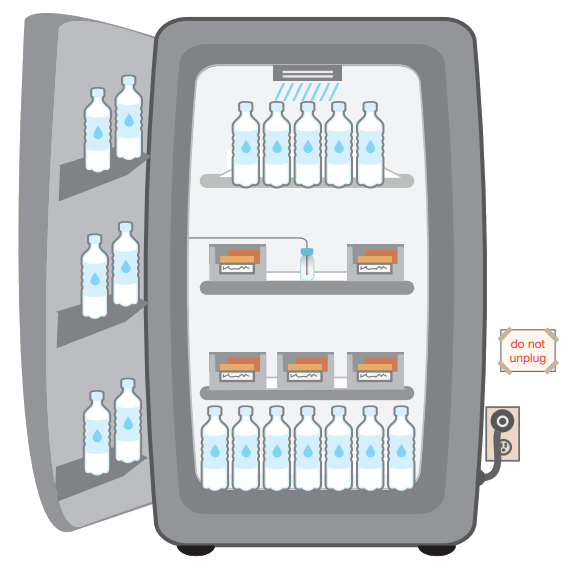
Place water bottles on the top shelf and floor and in the door racks. Putting water bottles in the unit can help maintain stable temperatures caused by frequently opening and closing unit doors or a power failure.
Water bottles are not recommended for use with certain pharmaceutical-grade and purpose-built units. For such units, follow the manufacturer’s guidance.
- Any staff who hears an alarm or notices a temperature excursion on the DDL should notify the primary or alternate vaccine coordinator immediately or report the problem to their supervisor.
- Notify staff by labeling exposed vaccines “DO NOT USE” and placing them in a separate container apart from other vaccines (do not discard these vaccines).
Regular Maintenance of Vaccine Storage Units and Temperature Monitoring Devices
Storage units and TMDs need regular maintenance to ensure proper operation.
- Conduct routine maintenance for all vaccine storage units and related equipment so that your equipment functions at maximum efficiency.
- Check seals and door hinges.
- Clean coils and other components per manufacturer direction.
- Defrost manual-defrost freezers when the frost exceeds either 1 cm or the manufacturer’s suggested limit. Follow the manufacturer’s instructions. While defrosting, store vaccines temporarily in another unit with appropriate freezer temperatures.
- Clean the interior of each unit to discourage bacterial and fungal growth. Do so quickly to minimize the risk of a temperature excursion.
- Test any backup generator quarterly and have it serviced annually.
Troubleshooting Equipment Problems
Adjusting Storage Unit Temperatures
Storage unit temperatures may need to be adjusted over time. In some situations, thermostats may need to be reset in summer and winter, depending on room temperature.
Temperature adjustments should be:
- Made by the primary or alternate vaccine coordinator, based on information from the TMD and temperature monitoring log
- Done at a time that is not during a busy workday when the unit door is being frequently opened and closed
Remember that temperatures within any storage unit will vary slightly, even with normal use. Therefore, before making any adjustment:
- Confirm the unit is securely plugged into a power source.
- Check the temperature inside the storage unit.
- Wait 30 minutes, without opening the door, to allow the temperature to stabilize and then check it again to determine if the thermostat should be adjusted.
If you believe there could be an issue with your TMD, use your backup device to confirm the temperature.
If you confirm that an adjustment is needed:
- Refer to the owner’s manual for detailed instructions. 2. Make a small adjustment toward a warmer or colder set
- Make a small adjustment toward a warmer or colder setting by turning the thermostat knob slowly to avoid going outside the correct temperature range.
- Once the adjustment is made, allow the temperature inside the unit to stabilize for 30 minutes without opening the door
- Recheck the temperature.
- . Repeat these steps as needed until the temperature has stabilized between 2° C and 8° C (36° F and 46° F) for a refrigerator or between -50° C and -15° C (-58° F and +5° F) for a freezer.
- Consider placing additional water bottles in the unit to help improve temperature stability.
Do not leave vaccines in a storage unit that does not maintain temperatures within the recommended range. If you are unable to stabilize the temperature in your unit within the required range, or temperatures in the unit are consistently at the extreme high or low end of the range, your vaccine supply is at high risk. Use your SOPs to identify an alternative unit with appropriate temperatures and sufficient storage space until the primary unit can be repaired or replaced.
Repeated Alarm Alerts
If the temperature alarm goes off repeatedly, do not disconnect the alarm until you have determined and addressed the cause. Do basic checks of the unit door, power supply, and thermostat settings. If the alarm continues to trigger or the temperature remains out of range, transfer vaccines to a backup unit as directed by your SOPs. A repair technician should check your equipment to determine the need for repair or replacement.
** If you are using a combination storage unit, note
that adjustments to the freezer temperature can adversely affect the refrigerator compartment temperature, possibly resulting in frozen vaccines in the refrigerator.






